
New Discoveries about the Nucleus of Atoms 2
As mentioned before, due to the symmetry and absorption of protons by the electron shell of neutrons in the helium nucleus, the following results could be obtained. According to the figures and the resultant of the force vectors, it could be said that each proton absorbs two neutrons and repels the other proton. In a way, the resultant of these forces is zero, so our structure is stable.
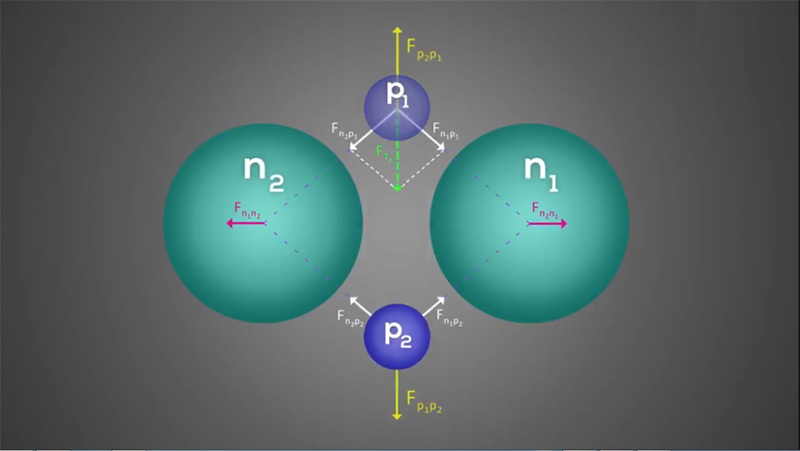
Since the effect of protons on neutrons is less than that of protons on protons, so the free space between two neutrons is almost less than the free space between two protons. Approximately, it could be said that if the free space between two neutrons is equal to the size of a proton, the free space between two protons is about a few protons. In fact as the repulsion between two protons that is equal to the absorption between an electron and a proton, is much more than the absorption between the neutron and the proton, so it could be concluded that the free space between two neutrons is about a proton and the free space between a proton and another proton is about two and a half protons. Therefore, we can say the previous figure is the final resultant effect of neutrons on neutrons, protons on protons, and neutrons on protons. And the free space between two protons will be about two and a half times of the distance between two neutrons. In fact, it could be said that the resultant of all attraction and repulsion forces are zero, and in this case, the nucleus of helium has the highest balance and stability. So the nucleus of helium is a symmetric nucleus, and we could place the axes of symmetry in any direction we want. This particular shape itself could explain why the helium is an inert gas.
Explanation of the structure of the helium nucleus
Although in the helium nucleus, neutrons and protons are facing each other pairwise, but in terms of height, protons are lower than neutrons. This arrangement of neutrons and protons in the helium nucleus is a logical, balanced, symmetrical structure with sufficient stability for its nucleus. So, its structure could be explained by considering Coulomb force as follows: Given that each neutron is composed of an electron and a proton and the outer shell of neutron is made of electron, it could be said that the outer shell of a neutron has a negative electric charge or create a negative charge environment around itself and protons can be absorbed by the neutron shell and be attracted towards it.
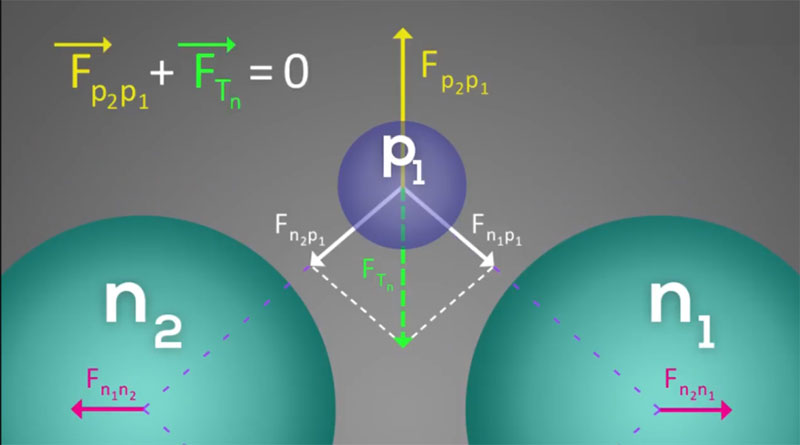
In fact, each proton is absorbed by two neutrons and repulses by the facing proton. In fact, each proton is affected by three forces, which include two attractions and one repulsion force, and since the resultant of these forces is zero and it is enclosed between them, we could say that each proton is fixed at a specific point. So, the nucleus of atom will have long-term stability. It should be noted that the repulsive force between two protons is greater than of two neutrons, which gives a rhombic shape to the helium nucleus from the top view.
Notice:
The amount of Coulomb force between protons and neutrons is very small, but the model of placement of neutrons and protons makes the nucleus very stable.
Explanation of the creation of nuclei heavier than helium
Based on the structure of the helium nucleus, to create the next elements, we should add one proton and one neutron respectively. If we continue this process of adding protons and neutrons, we will reach the hexagonal element, carbon. But since the structure of the nucleus must be a dense, continuous, steady, compact and stable structure, this process cannot be continued. Therefore, the carbon structure with 6 protons and 6 neutrons is the basis of heavier elements and the process of adding a proton and a neutron cannot be continued.
Explanation of the creation of nuclei heavier than carbon
As you can see in the figure, the structure of the heavier elements is such that the nuclei of the heavier elements are formed by the addition of neutrons and protons to the hexagonal ring of carbon.
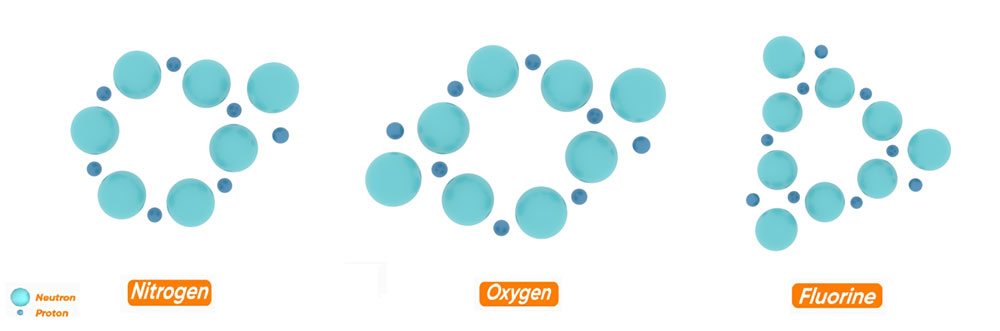
But this addition of neutrons and protons is so that besides this six-sided ring, three five-sided rings are formed symmetrically. Thus, one side of each pentagon and one side of the central hexagon are common.
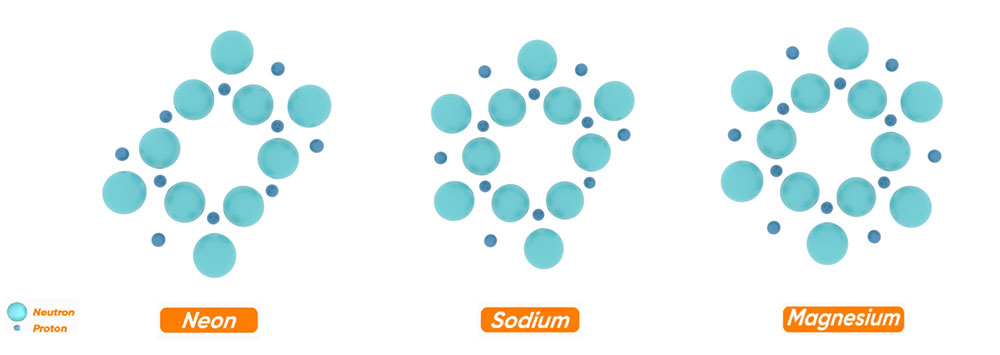
It should be noted that in the process of forming the nucleus of elements, the adding of neutrons and protons is in such a way that the nucleus of the desired atom obtains the most symmetrical and balanced structure.
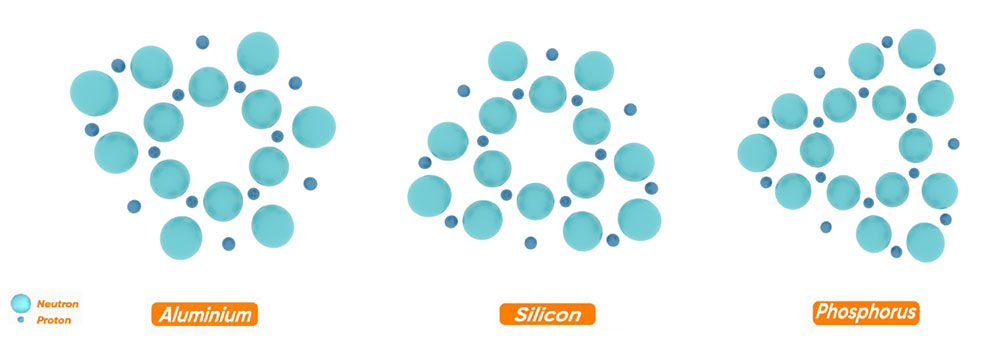
The adding of neutrons and protons continues until the nucleus of the argon element with 18 neutrons and 18 protons with 4 rings is formed. Therefore, the structure of the argon nucleus can be considered as a basis for the formation of nuclei of heavier elements.

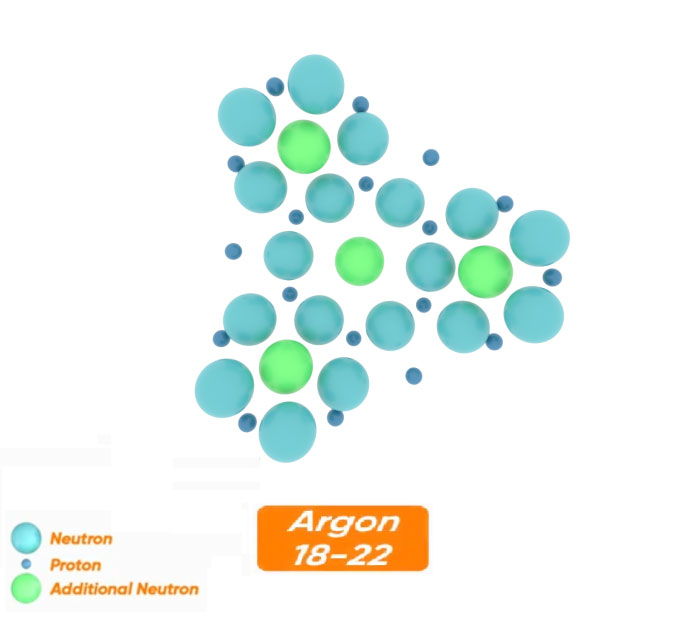
For example, let's look at the most abundant stable isotope of argon, which has 4 additional neutrons. The shape of the argon nucleus 18-22 is such that each of the 4 additional neutrons is placed inside one of the 4 rings and creates a symmetrical and stable structure. This shows the correctness of this structure.
Explanation of the creation of nuclei, heavier than argon
In fact, the argon with 18 protons and 18 neutrons is a hexagonal ring and 3 pentagonal rings as the first completed layer in nuclei. The krypton with 36 protons and 36 neutrons has 2 complete layers that the second layer is placed on the top of the first one at a proper angle. In fact, for elements heavier than the argon, the structure of the nucleus is completed in such a way that the subsequent neutrons and protons go to the higher layer and rotate proportion to the central axis, first forming the hexagon of the second layer and then the same forming process as the first layer continues until it reaches the krypton.

After krypton, it is time to form the third layer, which is the same as the first layer in terms of placement angle. The element which its third layer is completed is the xenon with 54 protons and 54 neutrons. Then the fourth layer begins to form and we reach the hafnium with 72 protons and 72 neutrons. These layers are placed on the structure of the argon nucleus at a proper angle so that it has the most balanced and symmetrical state. This trend continues for heavier elements as well.
For instance, consider the gold with atomic number 79. The same element that Mr. Rutherford used in his famous experiment that led to discovering of existence of a nucleus in the center of atom. The nucleus of this element is composed of 4 complete layers similar to argon and 1 incomplete layer of 7 protons and 7 neutrons. The gold has 39 additional neutrons in its stable isotope, which are located in the central cavity of the hexagons and pentagons, and the spaces between the two protons in all layers. As you can see, the gold nucleus has sufficient symmetry and consistency and spherical shape, which is in complete accordance with the results of the Rutherford experiment.
Notice:
As mentioned, the amount of force between neutrons and protons in the nucleus is low and their permanence is due to the model of placement of protons and neutrons. The question is:
“What is the source of so much energy generated by nuclear fission?”
Indeed, in the disintegration of the nuclei, the main energy is related to the rotation of the nuclei, which are spinning around themselves at a fraction of the speed of light. In other words, as nuclei spin around themselves at a fraction of the speed of light, when a nucleus breaks, neutrons and protons, and a number of the photons that make up these particles, separate from the nucleus at a similar speed. In fact,




